Technology
Patients with cancer-like symptoms or suspicious laboratory findings may be referred for radiological investigations followed by a biopsy for histopathological examination (HPE) which is the standard of care (SoC) to rule out cancer.
However, several patients (e.g., 50% - 80%, depending on the cancer type) who undergo a biopsy are diagnosed with non-cancerous (benign) conditions that may not present significant health risks.
Thus a significant number of patients without cancer have to undergo an invasive procedure that is associated with risks of pain, soreness, bleeding, and infection.
In some patients, some of these risks may be higher and so tissue sampling may not be possible. These patients have a risk of their cancer being missed; a risk which also applies to cases where the tissue sample may be insufficient. In such cases, a repeat tissue sampling procedure may be considered but may not always be possible.
There are two largely unmet needs for the benefit of patients suspected of cancer
To identify patients who are more likely to have cancer and prioritize them for a biopsy (i.e., ‘triaging’). This can reduce the need for (and risks of) invasive procedures in patients who are less likely to have cancer.
A safe and reliable non-invasive test, which can provide diagnostically relevant information to support clinical decision-making in cases where there are challenges to tissue sample-based diagnosis.
About Trublood™
- Trublood™ is an advanced non-invasive liquid biopsy test that provides diagnostically relevant information to support clinical decision-making.
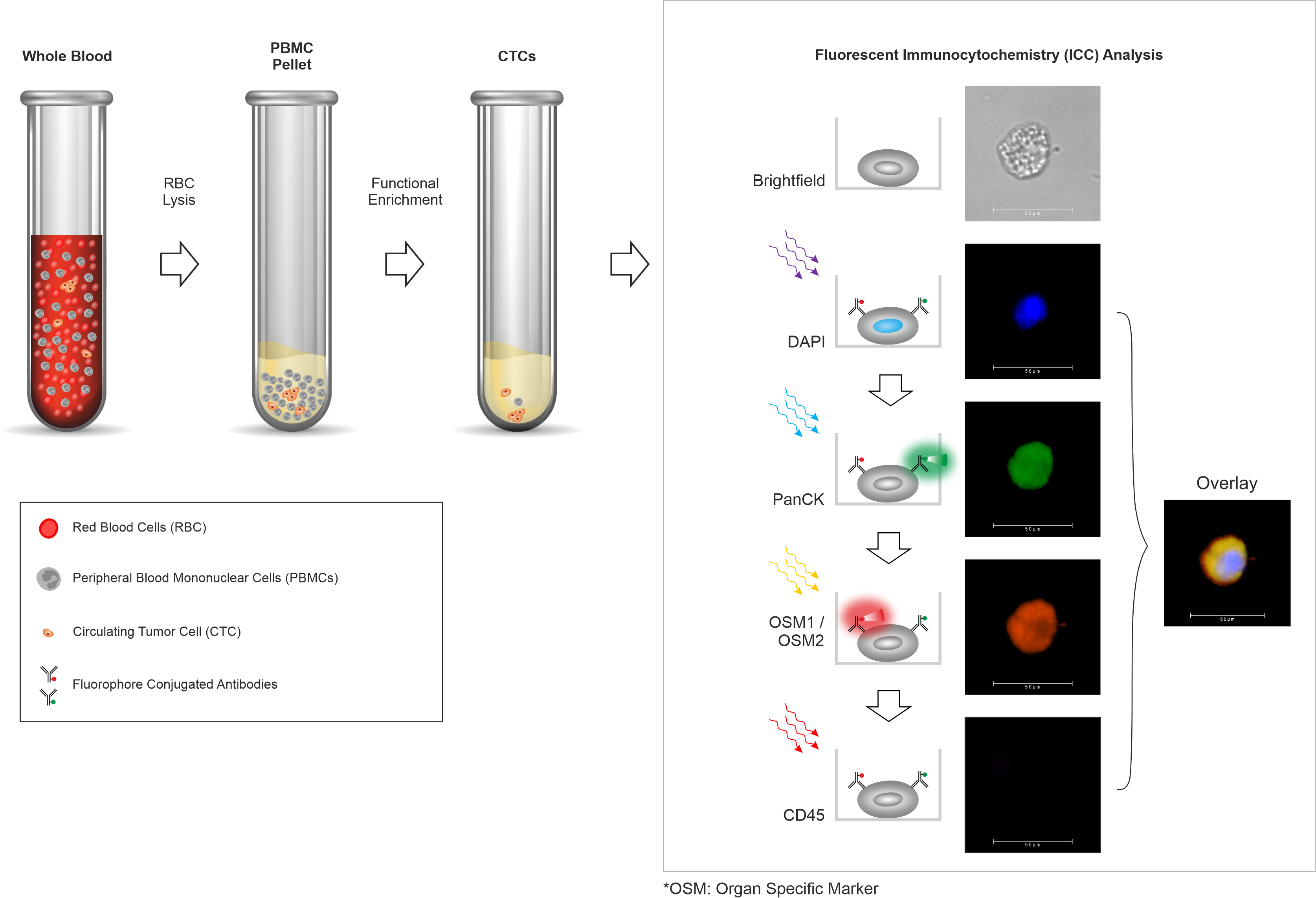
- Trublood™ evaluates blood samples to detect ‘Circulating Tumor Cells’ (CTCs), which are cancer cells shed by a malignant tumor into the blood.
- Trublood™ identifies CTCs (confirms their malignant identity) based on hallmark properties of cancer as well as the presence of cancer-associated biomarkers.
- A positive Trublood™ indicates that cancer might be present and the patient may benefit from follow-up or other investigations.
Reliable Technology
Trublood™ detects CTCs, which are known to be present (and detected ubiquitously) in most solid tumors. Prior clinical studies including our own have shown that CTCs are detectable even at early stages including Stage 0 (in situ cancer) as well as Stage I and Stage II (invasive cancers). CTCs are rare among individuals without cancer or those with non-cancerous conditions. Thus, CTCs have high accuracy for cancer detection.
Clinical Performance
The technology that powers Trublood™ has been developed and validated by analysis of more than 40,000 samples in large cohort studies.
Advantages
The ubiquity of CTCs in cancers translates into a potentially low risk of false negatives and missing cancers. The high cancer-specificity of CTCs translates into a lower risk of false positive findings in patients without cancer.
Trublood™ is currently available for the following cancer types:
- Trublood™ Test is not meant to replace or as an alternative to SoC cancer diagnosis.
- Positive finding on Trublood™ is not a confirmatory diagnosis of cancer. CTCs may be detected in individuals without cancer owing to the interplay of carcinogenesis and the anticancer immune mechanisms. CTC detection may also precede clinical or radiological manifestation by several months or years.
- Negative finding on Trublood™ is not confirmatory for the absence of cancer. The mechanism of CTC shedding by tumors into blood is not completely understood and hence CTCs may not always be present in blood. CTCs are whole cells and their distribution in circulating blood is dissimilar to other (‘soluble’) tumor analytes such as proteins and nucleic acids. CTCs exhibit diverse morphological characteristics, not all of which may be amenable to detection.
- Trublood™ findings must be interpreted by a clinician in conjunction with all other clinical or radiological evidence and diagnostic findings.
- Trublood™ test is not intended for detection of cancers in asymptomatic individuals.
- The suitability of Trublood™ testing as well as its potential benefits and risk must be evaluated by the treating clinician individually for each patient.